What Type of Energy Is Stored in a Battery?
From the smartphone you carry every day to the electric vehicle charging in your driveway, batteries quietly power much of modern American life. They’re so common that it’s easy to take them for granted. Yet inside that compact metal or plastic shell, a carefully controlled process is constantly at work.
This guide breaks down what’s really happening inside a battery. We’ll explain what type of energy a battery stores, why that energy exists in the form of chemical potential, and how it’s converted into the electrical power your devices rely on. Whether you’re preparing for wildfire-related outages in California or heading into the Rockies with portable gear, understanding how your energy source works is a practical step toward greater independence and reliability.
What Is Energy?
At its most basic level, energy is the ability to make something happen. In everyday terms, that might mean turning on a light, cooking dinner, or driving a car across town. Energy doesn’t appear out of nowhere. Instead, it exists in different forms and is constantly shifting from one state to another as we use it.
Types of Energy
Energy is usually grouped into two broad categories: potential energy, which is stored and waiting to be used, and kinetic energy, which comes from motion. A simple way to picture this is a roller coaster paused at the top of a hill at a theme park like Six Flags. At the peak, it holds stored energy. Once it drops, that stored energy becomes motion. Within these two categories, several specific forms of energy play an important role in how modern technology works.
Mechanical energy refers to the combined energy of motion and position within an object. Anytime something moves, lifts, spins, or applies force, mechanical energy is involved. When a portable power station runs an electric motor or powers a tool on a construction site, electrical energy is being converted into mechanical energy, allowing the equipment to perform real, physical work.
Thermal energy, commonly known as heat, comes from the movement of atoms and molecules inside a material. As that motion increases, so does the amount of heat produced. This is why a laptop often feels warm during long gaming or intensive work sessions; some of the energy is converted into heat instead of useful output. Modern, high-quality batteries are engineered to limit this heat loss, helping more energy reach your devices rather than warming the surrounding space.
Chemical energy is energy stored within the bonds of chemical compounds. It’s one of the most widely used forms of energy in everyday life, found in food, fuel, and modern battery systems. From the breakfast you eat to the gasoline that powers a classic Ford Mustang, chemical energy remains stored until a reaction releases it. Inside a battery, this energy stays in a stable state until a controlled chemical reaction allows it to be converted into usable power.
Electrical energy comes from the movement of electrons through a conductor. It’s the form of energy most commonly used in everyday American life, powering homes, offices, and infrastructure through the electrical grid. Because electricity is difficult to store directly in large amounts, batteries play a crucial role by converting electrical energy into chemical energy that can be stored safely and used later.
Understanding how these forms of energy relate to each other is essential to understanding batteries. A battery’s value lies in its ability to move energy back and forth between chemical and electrical states, allowing power to be stored, transported, and used on demand.
Energy Conversion and Storage
One of the core principles of physics, known as the First Law of Thermodynamics, states that energy cannot be created or destroyed; it can only change form. Storage is what makes this transformation practical in real life. For example, solar panels convert sunlight into electricity during the day. But when the sun sets over the Arizona desert, that energy doesn’t disappear. Instead, it must be stored in another form so it can be used later. Batteries fill this role by acting as an energy reservoir, capturing electricity and holding it as chemical energy until it’s needed again.
What Type of Energy Is a Battery?
It’s common to think of a battery as a container filled with electricity, but that’s not how it works. If you could open a battery, you wouldn’t find electricity stored inside it. Instead, you’d see a carefully designed chemical system built to store energy in a stable, controlled way.
1. Energy Stored as Chemical Potential Energy
A battery stores chemical potential energy. That energy is held inside the materials that make up the battery, mainly the anode, the cathode, and the electrolyte between them. It’s called potential energy because it’s stored, not active. When the battery is charged, those materials sit in a higher energy state and naturally want to react. The structure of the battery keeps that reaction controlled, so the energy is released slowly and safely only when the battery is connected to a device.
2. Output in the Form of Electrical Energy
When you connect a device like a phone or a lamp to a battery, you complete a circuit. That connection allows a chemical reaction to begin inside the battery. As the reaction happens, electrons are released and start moving through the wire and into the device. This movement of electrons is electrical energy, and it’s how the battery turns stored chemical energy into usable power. The process continues steadily until the chemicals inside the battery reach a more stable state or the circuit is broken.
3. Different Types of Batteries and Energy Storage
While all batteries rely on the same basic idea, storing chemical energy and releasing it as electricity, the materials used inside them can vary a lot. Those material choices affect how much energy a battery can hold, how heavy it is, how long it lasts, and where it makes the most sense to use.
Lithium-ion batteries
Lithium-ion technology has fundamentally changed how backup power works by removing many of the limitations tied to older battery systems. Traditional lead-acid batteries can store energy, but they’re heavy, take up a lot of space, and usually require fixed installation, which makes them a poor fit for people who need flexibility or portability. Lithium-ion batteries solve this by delivering high capacity in a much smaller, lighter footprint, without locking users into a permanent setup. This technology encompasses different types of lithium batteries, such as Lithium Iron Phosphate (LFP) and Nickel Manganese Cobalt (NMC), each offering unique benefits for energy density and cycle life.
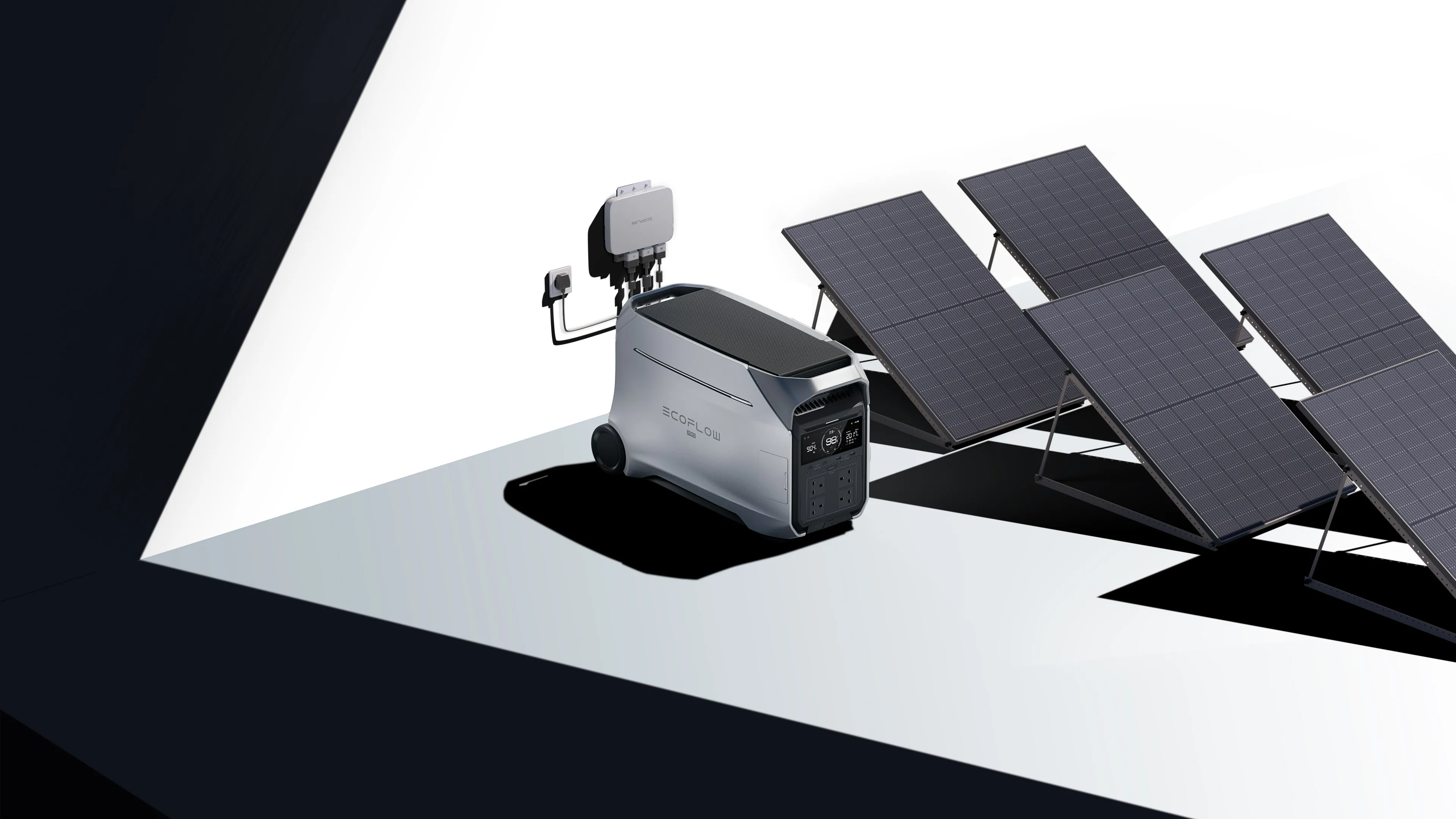
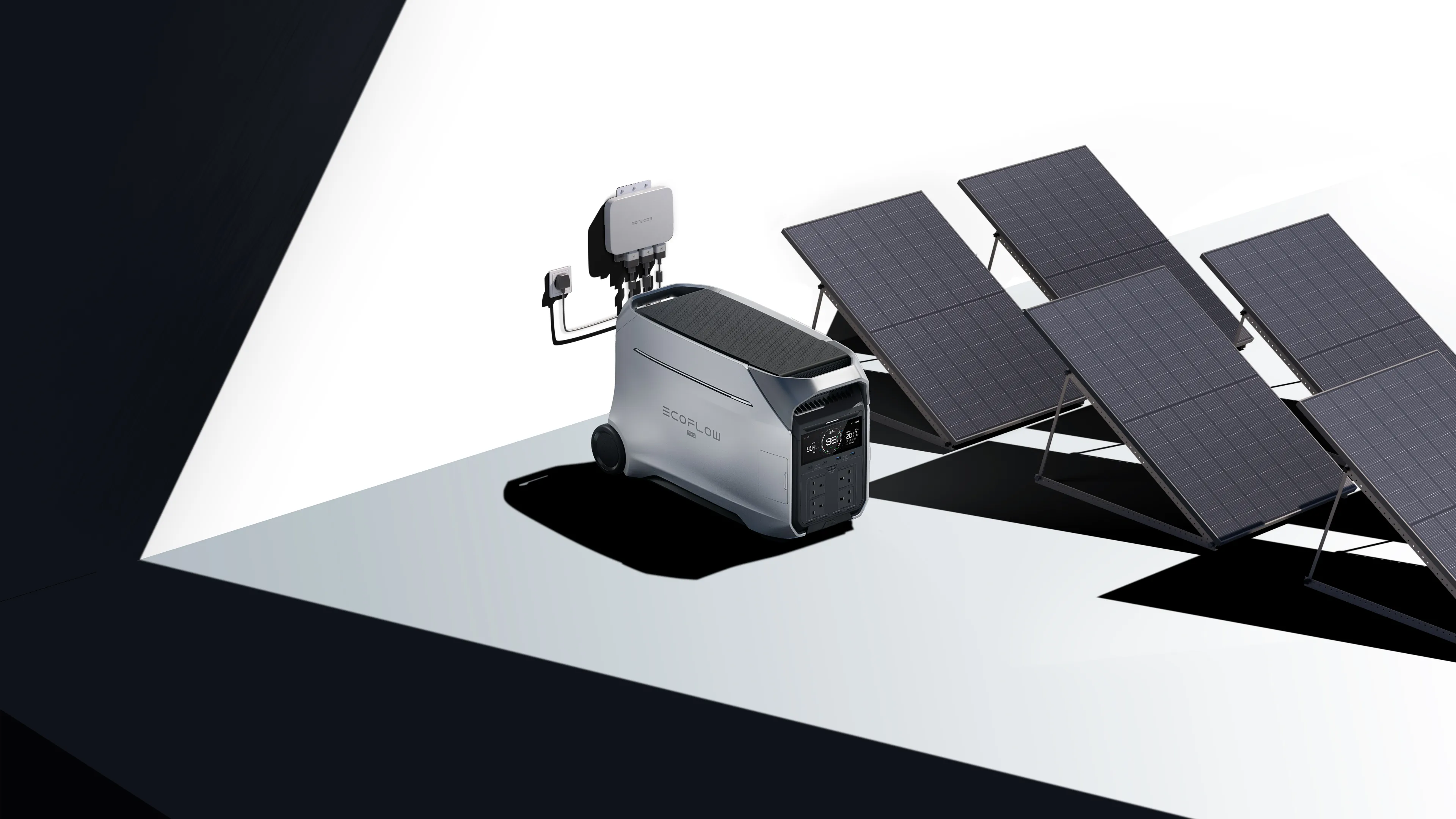
That shift is why portable lithium power stations have become the preferred choice for modern backup and mobile power. Their high energy density allows several kilowatt‑hours of usable capacity to be packed into a single unit that can be moved, stored, or deployed wherever power is needed. A good example is the EcoFlow DELTA Pro 3 Portable Power Station, which offers around 4 kWh of usable energy and up to 4000 W of continuous AC output, with short‑term peaks up to 6000 W using X‑Boost technology—enough to support many household appliances and essential systems during outages.
Lead-acid batteries
Lead-acid batteries are one of the oldest battery types still in widespread use. Most gasoline-powered vehicles in the U.S. rely on them to start the engine. They are durable, inexpensive, and well understood, but they are also heavy and inefficient compared to newer options. Because they store less energy per pound, they tend to be bulky and are best suited for basic, short-term tasks.
Nickel-metal hydride (NiMH) batteries
NiMH batteries sit between older and newer technologies. They are commonly found in older hybrid vehicles and rechargeable household batteries. While they are more environmentally friendly than some older chemistries and more stable than early battery designs, they generally don’t store as much energy or hold a charge as efficiently as lithium-ion batteries.
How Do Batteries Store Energy and Produce Electricity?
Inside a battery, energy storage and release happen through controlled chemical reactions. While the process is carefully engineered, the basic idea is straightforward: energy is stored by rearranging chemical components and released when those components are allowed to react again.
1. Chemical Reactions Inside a Battery
Every battery contains two main parts called electrodes: the anode (negative) and the cathode (positive). These are separated by an electrolyte that allows ions to move between them. When a battery is charged, ions are pushed from the cathode toward the anode, creating a stored chemical imbalance that holds energy.
2. Charging Process (Storing Energy)
When you plug a battery or power station into a standard 120-volt wall outlet, external electricity forces those ions back into position at the anode. This input energy changes the chemical arrangement inside the battery and stores energy in the process. At this stage, the battery is holding energy but not releasing it.
3. Stored State (Energy at Rest)
Once charging is complete, the battery can sit unused in a garage, closet, or backpack while holding that energy. Modern batteries are designed to lose very little charge over time, a feature known as low self-discharge. As long as nothing is connected, the chemicals remain stable and the stored energy stays locked inside.
4. Discharge Process (Producing Electricity)
When a device is turned on, the stored chemical energy is released. Ions move back toward the cathode inside the battery, while electrons are pushed through the external circuit, passing through the device before returning to the battery. That movement of electrons is what we recognize as electricity powering lights, tools, or electronics.
5. Charge and Discharge Cycles
Each full use of this process, from charging to discharging, counts as one cycle. Over time, repeated cycles cause gradual changes inside the battery’s materials. This natural wear is why batteries slowly lose capacity after years of regular use, even when they’re well-maintained.
Practical Applications: How Batteries Power Everyday Devices
From high-rise apartments in New York City to off-grid homes and ranches in Montana, batteries play a quiet but essential role in solving everyday power needs. Their ability to store energy and deliver it on demand makes modern life more flexible and less dependent on constant access to the grid.
1. Portable Electronics
The rise of smartphones, laptops, and tablets would not have been possible without compact, high-density batteries. These devices need a steady supply of power in a form small enough to fit into a pocket, backpack, or briefcase. Batteries make it possible to work, communicate, and stay connected without being tied to a wall outlet.
2. Electric Vehicles (EVs)
Electric vehicles rely entirely on large battery systems to operate. Inside an EV, stored chemical energy is converted into mechanical energy that turns the wheels. This process is highly efficient compared to traditional engines and plays a growing role in reshaping how Americans commute, especially in urban and suburban areas.


3. Renewable Energy Storage
Solar and wind power don’t produce energy on demand; they produce it when conditions allow. Batteries bridge that gap by storing excess energy generated during sunny afternoons or windy days. That stored energy can then be used later, such as powering a home overnight after the sun sets in Nevada or during calm weather when wind generation slows.
4. Backup Power Systems
For many households across the U.S., backup power has shifted from a nice-to-have feature to a practical necessity, especially as outages become more common. That said, most families don’t need a massive system designed to run an entire estate. What matters more is finding the right balance between usable power and everyday portability. This is where compact solutions like the EcoFlow DELTA 3 Ultra Series Portable Power Station stand out. Built with high-density lithium-ion cells, it delivers 3072Wh of reliable energy in a size that’s easy to store in a hallway closet or load into a car for a weekend at a national park. It’s well-suited for keeping essentials like lighting, internet equipment, and small appliances running during an outage, turning stored chemical energy into dependable, real-world reassurance.
5. Smart Home Devices and IoT Applications
Smart home technology depends heavily on batteries. Sensors, security cameras, smart locks, and other connected devices often run continuously without being hardwired into a home’s electrical system. Small, efficient batteries make it possible for these devices to operate around the clock, providing convenience and security without complex installation.
Common Misconceptions About Battery Energy Storage
As batteries move beyond small electronics and become part of everyday home energy systems, misunderstandings about how they work have become more common. Clearing up these myths helps people make better decisions, especially when batteries are used for home backup power, renewable energy storage, or long-term reliability.
1. All Batteries Use the Same Technology
One of the most widespread misconceptions is that all batteries function the same way. While they all store energy chemically, the materials inside them make a major difference. Lead-acid batteries, for example, are dependable and affordable but heavy and relatively short-lived. Lithium-based batteries vary even further. Some chemistries prioritize high energy density and lighter weight, while others focus on safety and long service life. The best choice depends on how the battery will be used, whether portability, longevity, or durability matters most.
2. Batteries Lose Their Charge Quickly When Not in Use
Many people associate batteries with the frustration of finding a dead flashlight after months of storage. While older alkaline batteries were prone to leaking and rapid self-discharge, modern lithium systems behave very differently. High-quality lithium batteries lose only a small amount of charge over time, often just a few percent per month. This makes them well-suited for emergency use, where a battery may sit unused for long periods before being needed.
3. Charging to 100% or Draining to 0% Is Always Harmful
Older battery advice often warned against fully charging or deeply draining devices. While extreme conditions can still stress battery cells if maintained for long periods, modern batteries are far more forgiving. Most include built-in protection systems that manage charging speed, limit overcharging, and prevent complete discharge. These systems help protect the internal chemistry and extend the battery’s usable life without requiring constant attention from the user.
4. Batteries Are Safe and Don't Require Maintenance
Modern lithium batteries are designed to be safe and easy to use, but they still need basic care. They aren’t fragile, yet they aren’t immune to misuse either. Consistent exposure to extreme heat, such as leaving a battery in a locked car during an Arizona summer, can shorten its lifespan by damaging the internal chemistry. Maintenance today is less about physical upkeep and more about software. Many modern battery systems rely on built-in management software to control charging, temperature, and safety limits. Keeping that firmware up to date helps ensure the battery continues to operate within safe parameters and performs as intended over time.
5. Batteries Have No Environmental Impact
It’s easy to assume that batteries are environmentally neutral because they don’t produce emissions during use. In reality, producing battery materials requires mining and processing that carry environmental costs. That said, longevity matters. A battery that lasts many years reduces the need for frequent replacements, lowering overall waste and demand for raw materials. Choosing durable systems with long cycle life helps reduce their environmental footprint over time.
Conclusion
A battery is more than a simple power source; it’s a practical system that connects stored chemical energy to the electricity we rely on every day. Understanding that batteries store chemical potential energy helps explain why their design, materials, and management systems matter so much in real-world use. Whether you’re preparing for winter outages, keeping essentials running during a storm, or simply charging everyday devices, choosing the right battery technology makes a noticeable difference in reliability and lifespan. From emergency preparedness to daily convenience, well-designed batteries allow energy to be stored safely and used when it’s needed most, wherever life happens to take you.
FAQ
1. Is the Battery a DC or AC?
A battery produces direct current (DC), where energy flows in a single direction, allowing it to store and release power. Understanding the difference between AC and DC is key, as household outlets use alternating current (AC), which periodically reverses direction. That’s why many portable power stations and home backup systems include a built-in inverter to convert DC power from the battery into AC power that standard appliances can use.
2. Are Batteries a Form of Renewable Energy?
No, batteries themselves are not a source of renewable energy. They don’t generate power; they store it. A battery’s environmental impact depends entirely on how it’s charged. When paired with renewable sources like solar or wind, batteries become essential tools for storing clean energy and making it available around the clock.
3. Are batteries useful without solar panels?
Yes, many people use batteries even without solar. One common use is managing electricity costs by charging a battery during off-peak hours when rates are lower and using that stored energy during peak pricing periods. Others keep batteries on hand simply for backup power during outages, offering peace of mind regardless of how the battery is charged.
4. What Is the Best Home Battery Backup System?
The right home battery backup depends on your needs. For homeowners who want both portability and high output, the EcoFlow DELTA Pro 3 is a top choice. Its expandable design allows you to start with essential backup and add extra batteries over time, making it capable of supporting everything from critical devices to near whole-home power during outages.
5. How Long Does a Home Backup Battery Last?
Battery runtime depends on both the battery’s capacity and what you’re powering. A 1,000Wh battery can run a 100-watt device for about 10 hours, but a high-power appliance like a coffee maker or space heater will drain it much faster. Estimating usage comes down to understanding the wattage of your devices and matching them to the battery’s capacity.
For press requests or interview opportunities, reach out to our media team
media.na@ecoflow.com